The conductivity ụlọ ọrụ usoro nke electrodes na-pụrụ iche na-eji maka nha nke conductivity uru nke dị ọcha mmiri, ultra-ọcha mmiri, mmiri ọgwụgwọ, wdg Ọ bụ karịsịa adabara conductivity nha na thermal ike osisi na mmiri ọgwụgwọ ụlọ ọrụ.A na-egosipụta ya site na nhazi cylinder okpukpu abụọ na ihe titanium alloy, nke nwere ike ime ka ọ bụrụ ihe na-eme ka ọ bụrụ ihe na-esi na ya pụta.Ihe mgbochi infiltration na-eduzi elu ya na-eguzogide ụdị mmiri ọ bụla ma e wezụga fluoride acid.Akụkụ nkwụghachi ụgwọ okpomọkụ bụ: NTC2.252K, 2K, 10K, 20K, 30K, ptl00, ptl000, wdg nke onye ọrụ ahụ akọwapụtara.K = 10.0 ma ọ bụ K = 30 electrode na-anabata nnukwu akụkụ nke nhazi platinum, nke na-eguzogide acid siri ike na alkaline ma nwee ikike mgbochi mmetọ siri ike;a na-ejikarị ya eme ihe n'ịntanetị maka uru conductivity na ụlọ ọrụ pụrụ iche, dị ka ụlọ ọrụ na-ahụ maka nsị mmiri na ụlọ ọrụ na-eme ka mmiri dị ọcha.
| Na-adịgide adịgide nke electrode | 0.1 | 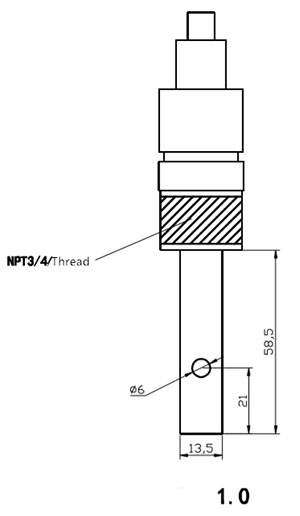 |
| Ike mkpakọ | 0.6MPa | |
| Oke nha | 0-200uS/cm | |
| Njikọ | 1/2 ma ọ bụ 3/4 Ntinye eriri | |
| Ihe onwunwe | 316L Titanium Alloy na Platinum | |
| Ngwa | Ụlọ ọrụ ọgwụgwọ mmiri |
Omume omumebụ ihe nleba anya nke ike mmiri iji gafee ọkụ eletrik.Ikike a metụtara kpọmkwem na ntinye nke ion na mmiri
1. Ndị a conductive ion si etisasịwo salts na inorganic ihe dị ka alkalis, chlorides, sulfides na carbonate ogige.
2. Ngwakọta ndị na-agbaze n'ime ion ka a na-akpọkwa electrolytes 40. Ka ion ndị ọzọ dị, otú ahụ ka conductivity nke mmiri dị.N'otu aka ahụ, ion ole na ole dị na mmiri, otú ahụ ka ọ na-eme ka ọ dị ntakịrị.Mmiri distilled ma ọ bụ deionized nwere ike ịrụ ọrụ dị ka ihe mkpuchi n'ihi uru conductivity dị ala (ma ọ bụrụ na ọ bụghị nke na-adịghị efu).Mmiri mmiri, n'aka nke ọzọ, nwere nnọọ elu conductivity.
Ions na-eduzi ọkụ eletrik n'ihi ụgwọ ha dị mma na nke na-adịghị mma
Mgbe electrolytes gbazere n'ime mmiri, ha na-ekewa n'ime cation (cation) na ebubo na-ezighị ezi (anion).Ka ihe ndị gbazere na-ekewa n'ime mmiri, ọnụọgụ nke ụgwọ ọ bụla dị mma na nke na-adịghị mma na-anọgide nhata.Nke a pụtara na n'agbanyeghị na conductivity nke mmiri na-abawanye na ions agbakwunyere, ọ na-anọgide na-anọpụ iche eletriki 2
Ịrụ ọrụ/Nguzogidebụ usoro nyocha nke a na-ejikarị eme ihe maka nyocha ịdị ọcha nke mmiri, nleba anya nke reverse osmosis, usoro nhicha, njikwa usoro kemịkalụ, na mmiri mkpofu ụlọ ọrụ.Nsonaazụ ntụkwasị obi maka ngwa ndị a dị iche iche dabere na ịhọrọ ihe mmetụta conductivity ziri ezi.Ntuziaka ekele anyị bụ akwụkwọ ntụaka na ọzụzụ zuru oke dabere na ọtụtụ iri afọ nke ndị isi ụlọ ọrụ na nha a.
Conductivity bụ ikike nke ihe na-eduzi ọkụ eletrik.Ụkpụrụ nke ngwaọrụ ji atụ conductivity dị mfe-a na-etinye efere abụọ n'ime ihe nlele ahụ, a na-etinye ike n'ofe efere (na-emekarị voltaji sine), na ugbu a na-agafe na ngwọta na-atụle.




























